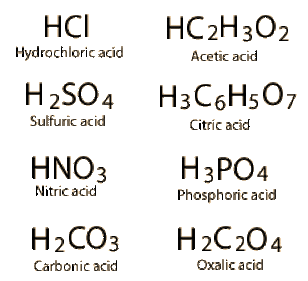Acids
What exactly is an acid? Don't feel too acidic if you aren't sure! Let's start off by listing some properties.
An acid is a chemical substance or molecule that can:
An acid is a chemical substance or molecule that can:
- Neutralize alkalis
- Neutralize bases to produce salt and water
- React with carbonates to produce a salt, water and CO2
- React with active metals to produce hydrogen
- Turn blue litmus to red
- Turn methyl orange to red
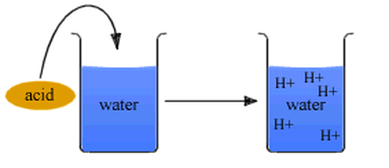
- Increase H+ ion concentration in a solution
In addition, they:
There are also 3 important theories that talk about important properties of acids. The 3 theories are summarized below but more elaborate information about all of them can be found here
1) Lewis Theory
2) Bronsted-Lowry Theory
3) Arrhenius Theory
There are many different acids that can be formed. Below are some common ones:
- Are good conductors of electricity in water
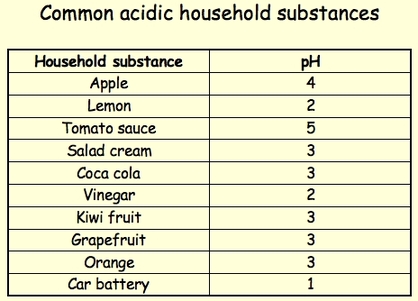
- Have a pH below 7
- Are usually corrosive or sour tasting
- Are strong electrolytes
- Have more hydrogen ions than hydroxide ions
There are also 3 important theories that talk about important properties of acids. The 3 theories are summarized below but more elaborate information about all of them can be found here
1) Lewis Theory
- States that an acid is an electron pair acceptor
2) Bronsted-Lowry Theory
- States that an acid is a proton (hydrogen ion) donor
3) Arrhenius Theory
- States that acids are substances which produce hydrogen ions in a solution
There are many different acids that can be formed. Below are some common ones:
Here are what some of the acids above are used for:
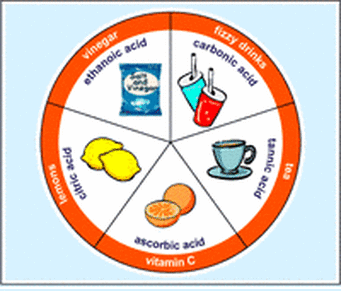
Some examples of acids used in our everyday lives include:
- Hydrochloric Acid - Is important for dissolving metals and can be found in human stomachs
- Sulfuric Acid - Helps produce fertilizers and is used in car batteries
- Nitric Acid - Helps produce fertilizers, medicine, and paint
- Acetic Acid - Is a solvent and helps produce rubber and plastic
- Citric Acid - Is in many fruits like oranges, lemons, and grapefruits
Some examples of acids used in our everyday lives include:
- Milk
- Sodas/Carbonated Drinks
- Orange/Lemon Juice
- Citric Fruits
- Tea
- Vinegar