pH & pOH
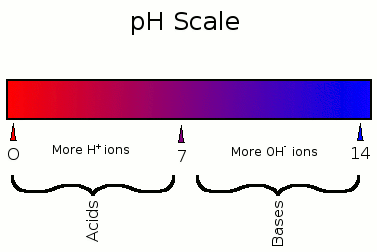
pH is a figure or unit that expresses the acidity or alkalinity of a solution using a scale with values from 0 to 14, with 7 being neutral, anything under 7 being an acid, and anything above 7 being a base/alkaline. pH is equal to -log 10^c, where "c" represents the hydrogen ion concentration in moles per liter. This is a very important equation that you will learn much more about later on. In addition, you will find 2 carefully marked pH scales showing specific characteristics about acids and bases below to give an overview about some characteristics of pH:
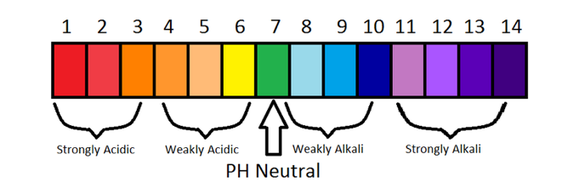
From these 2 pH scales, you can conclude a few things:
- pH of 1-3 is a strong acid
- pH of 4-6 is a weak acid
- pH of 7 is neutral
- pH of 8-10 is a weak base/alkali
- pH of 11-14 is strong base/alkali
- Acids have more H+ ions
- Bases have more OH- ions
pOH is fairly similar to pH, except instead of measuring the hydrogen ion concentration in moles per liter, it measures the hydroxide ion concentration in moles per liter. Since a substance won't always be fully acidic or fully basic, there will need to be a measurement for both. These two measurements must add up to 14: pH + pOH = 14. (e.g. pH of 5 means pOH of 9). The scale for pOH is the reverse of the pH scale.
The strength and concentration of acids and bases are typically confused and regarded as the same thing. However, they are two different concepts, with the two being reliant on the other. The strength of an acid or base has to do with its pH or pOH, which you just learned about above. This information can then be used to figure out the concentration of the acid or base, which is essentially the amount of hydrogen or hydroxide ions present, or the amount of ions present per unit volume of a solution.
This all relates back to the idea of strong acids and bases. Strong acids and bases will completely dissociate to form hydrogen and hydroxide ions, respectively, and weak acids and bases will only partially dissociate to form hydrogen and hydroxide ions, respectively. In terms of pH, if the strength or pH is lower, the higher the concentration (of hydrogen ions) will be. In terms of pOH, if the strength or pOH is lower, the higher the concentration (of hydroxide ions) will be. Equations about pH/pOH, strength and concentration can be found below:
**"[ ]" is equal to the concentration of"**
Using the equations above, you can figure out that a concentration of hydrogen ions that is equal to 0.1 M will have a pH of 1. You can also figure out that a concentration of 0.01 M will have a pH of 2. There's a pattern: if the concentration is in the form of 10^-x, the pH will be x.
A helpful video covering pH and pOH calculations can be found below.
A link for practice problems using these equations can be found at the bottom of this page or here
This all relates back to the idea of strong acids and bases. Strong acids and bases will completely dissociate to form hydrogen and hydroxide ions, respectively, and weak acids and bases will only partially dissociate to form hydrogen and hydroxide ions, respectively. In terms of pH, if the strength or pH is lower, the higher the concentration (of hydrogen ions) will be. In terms of pOH, if the strength or pOH is lower, the higher the concentration (of hydroxide ions) will be. Equations about pH/pOH, strength and concentration can be found below:
- pH = -log [H+]
- [H+] = 2nd Fn log [ -pH]
- pOH = -log [OH-]
- [OH-] = 2nd Fn log [ -pOH]
- pH + pOH = 14
- [H+][OH-] = 1x10-14 (1E-14)
**"[ ]" is equal to the concentration of"**
Using the equations above, you can figure out that a concentration of hydrogen ions that is equal to 0.1 M will have a pH of 1. You can also figure out that a concentration of 0.01 M will have a pH of 2. There's a pattern: if the concentration is in the form of 10^-x, the pH will be x.
A helpful video covering pH and pOH calculations can be found below.
A link for practice problems using these equations can be found at the bottom of this page or here