History
Acids and bases have been studied for hundreds of years. Our current understanding of acids and bases is based on the studies and contributions of many scientists, chemists, and biologists over the past 200 years or so. Below is a brief timeline of some important discoveries:
- 1865 - Introduction of antiseptic spray containing carbolic acid; marks beginning of modern antiseptic surgery
- 1869 - Nucleic acids are discovered and found in cell nuclei
- 1883 - Svante Arrhenius proposes that acids will produce hydrogen ions and bases will produce hydroxide ions when dissolved in water
- 1909 - The development of the pH scale allows scientists to define the acidity of a substance
- 1923 - Scientists expand on and revise the exact definitions of acids and bases, which translate into our definitions today
- 1933 - Scientists develop portable pH meters
- 1953 - James Watson, Francis Drick, and Rosalind Franklin study nucleic acid DNA, which is important towards today's biotech industry
- 1963 - Scientists discover acid rain in North America; pH measurements show that polluted rain is 100 times more acidic than regular rain
- 1980 - Silicon chip pH meters have no glass contents; now widely used in the food and cosmetic industries, along with pharmacies
- 2005 - Scientists develop super acids: more acidic than 100% sulfuric acid.
Now you will learn more about the three theories that describe acids and bases in more detail.
Let's start by reminding you of what those are: 1) 1) Arrhenius Theory; 2) Bronsted-Lowry Theory; and 3) Lewis Theory
All of these theories are named after the scientists that formulated them over one hundred years ago. Let's get into more detail.
First, the Arrhenius Theory, or Model, which it is also commonly referred to, was formulated by a Swedish Chemist named Svante Arrhenius in 1883. This states that an acid is a substance that contains hydrogen and also ionizes to produce hydrogen ions in an aqueous solution, and that a base is a substance that contains a hydroxide group and dissociates to produce hydroxide ions in an aqueous solution.
Let's take a look at what happens to an acid and a base according to the Arrhenius Theory:
Hydrochloric Acid: HCl (g) → H+ (aq) + Cl- (aq)
Sodium Hydroxide: NaOH (s) → Na+ (aq) + OH- (aq)
The Arrhenius Theory does not work for all acids and bases though, like ammonia and sodium carbonate, since they don't contain a hydroxide group, but they somehow still produce hydroxide ions in a solution.
Let's start by reminding you of what those are: 1) 1) Arrhenius Theory; 2) Bronsted-Lowry Theory; and 3) Lewis Theory
All of these theories are named after the scientists that formulated them over one hundred years ago. Let's get into more detail.
First, the Arrhenius Theory, or Model, which it is also commonly referred to, was formulated by a Swedish Chemist named Svante Arrhenius in 1883. This states that an acid is a substance that contains hydrogen and also ionizes to produce hydrogen ions in an aqueous solution, and that a base is a substance that contains a hydroxide group and dissociates to produce hydroxide ions in an aqueous solution.
Let's take a look at what happens to an acid and a base according to the Arrhenius Theory:
Hydrochloric Acid: HCl (g) → H+ (aq) + Cl- (aq)
Sodium Hydroxide: NaOH (s) → Na+ (aq) + OH- (aq)
The Arrhenius Theory does not work for all acids and bases though, like ammonia and sodium carbonate, since they don't contain a hydroxide group, but they somehow still produce hydroxide ions in a solution.
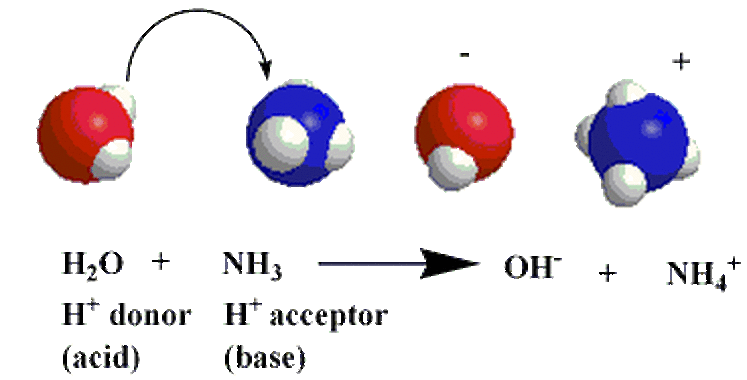
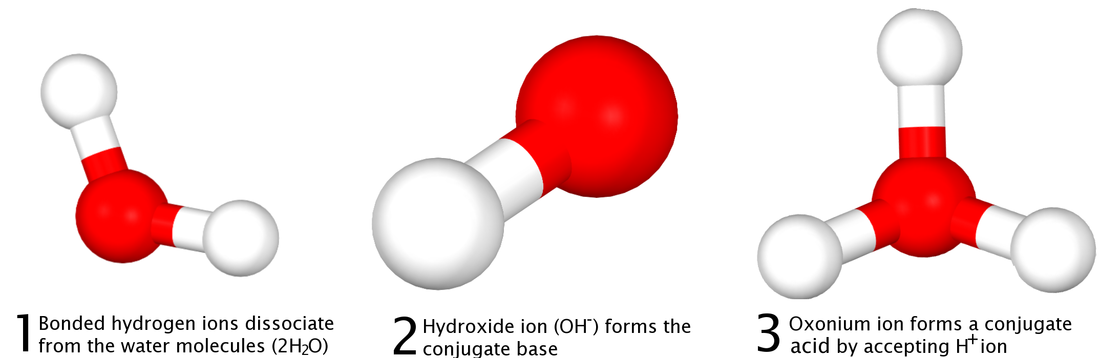
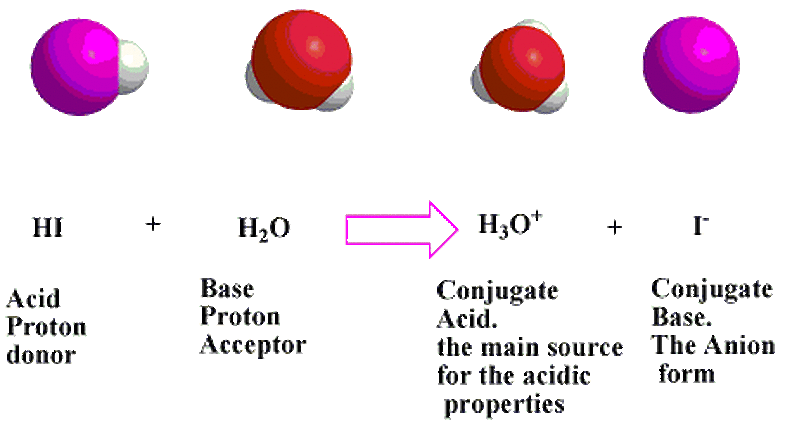
Next, the Bronsted-Lowry Theory, formulated by a Danish Chemist named Johannes Bronsted and an English Chemist named Thomas Lowry sometime in the 19th or 20th centuries. Their proposal was a more inclusive model of both acids and bases that was based a lot on hydrogen ions. Their theory states that an acid is a hydrogen ion donor and that base is a hydrogen ion acceptor.
Examples of this theory can be found here, as they are directly related to conjugate acids and bases. A specific example of a Bronsted-Lowry acid would be hydrogen fluoride and a specific example of a Bronsted-Lowry base would be ammonia. Water would be a Bronsted-Lowry acid and base. Two pictures diagramming the theory can be found below:
Examples of this theory can be found here, as they are directly related to conjugate acids and bases. A specific example of a Bronsted-Lowry acid would be hydrogen fluoride and a specific example of a Bronsted-Lowry base would be ammonia. Water would be a Bronsted-Lowry acid and base. Two pictures diagramming the theory can be found below:
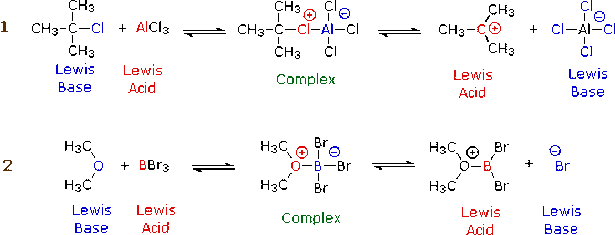
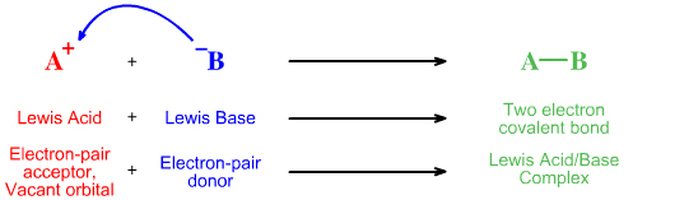
And finally, the Lewis Theory, which was an even more general model of acids and bases, was formulated by American Chemist, G.N. Lewis. You may know Lewis as the person who developed the electron-pair theory of chemical bonding and who also came up with the idea of Lewis Structures which keeps track of all the valence electrons in atoms and molecules.
His idea and theory about acid and bases kind of relates to what he already came up with. His theory states that an acid is an electron pair acceptor and that a base is an electron pair donor. Below are two pictures that diagram this theory:
His idea and theory about acid and bases kind of relates to what he already came up with. His theory states that an acid is an electron pair acceptor and that a base is an electron pair donor. Below are two pictures that diagram this theory: