Neutralization
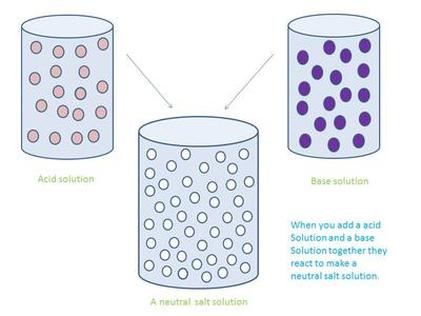
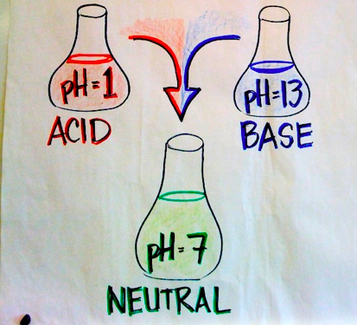
Neutralization is the act of making an acidic or basic substance chemically neutral, meaning a pH of 7. Mixing an acid with substances that are bases are examples of neutralization reactions. Neutralization can be backed up by acid and base theories. Neutralization reactions can make salts along with some other products. General and specific examples of these reactions can be found below:
1. Acid + Metal → Salt + H2 (hydrogen gas)
2HCl + Zn → ZnCl2 + H2
2HNO3 + Mg → Mg(NO3)2 + H2
2. Acid + Carbonate → Salt + H2O (water) + CO2 (carbon dioxide gas)
2HCl + Na2CO3 → 2NaCl + H2O + CO2
H2CO4 + MgCO3 → MgCO4 + H2O +CO2
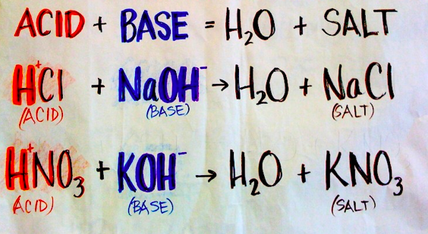
3. Acid + Base → Salt + H2O (water)
HCl (Hydrochloric acid) + NaOH → NaCl + H2O
H2SO4 (Sulfuric acid) + Ca(OH)2 → CaSO4 + 2H2O
HNO3 (Nitric acid) + KOH → KNO3 + H2O
4. Acid + Alkali → Salt + H2O (water)
As you can see, salt is produced in all of the neutralization reactions, some of which are known as double displacement reactions (examples 1 and 3). There are four main families of salts: chlorides, carbonates, sulfates, and nitrates. All of these except for carbonates, are commonly used in acid-base reactions. The salt produced in a reaction will depend on the acid that is being reacted with since a part of it will end up being part of the salt, as you can see in the reactions below example #3.
Water is also produced in a majority of these equations. Water is neutral and has a pH of 7, which makes sense since we are dealing with neutralization reactions. Some images below will help clarify things about neutralization reactions if you are lost:
1. Acid + Metal → Salt + H2 (hydrogen gas)
2HCl + Zn → ZnCl2 + H2
2HNO3 + Mg → Mg(NO3)2 + H2
2. Acid + Carbonate → Salt + H2O (water) + CO2 (carbon dioxide gas)
2HCl + Na2CO3 → 2NaCl + H2O + CO2
H2CO4 + MgCO3 → MgCO4 + H2O +CO2
3. Acid + Base → Salt + H2O (water)
HCl (Hydrochloric acid) + NaOH → NaCl + H2O
H2SO4 (Sulfuric acid) + Ca(OH)2 → CaSO4 + 2H2O
HNO3 (Nitric acid) + KOH → KNO3 + H2O
4. Acid + Alkali → Salt + H2O (water)
As you can see, salt is produced in all of the neutralization reactions, some of which are known as double displacement reactions (examples 1 and 3). There are four main families of salts: chlorides, carbonates, sulfates, and nitrates. All of these except for carbonates, are commonly used in acid-base reactions. The salt produced in a reaction will depend on the acid that is being reacted with since a part of it will end up being part of the salt, as you can see in the reactions below example #3.
Water is also produced in a majority of these equations. Water is neutral and has a pH of 7, which makes sense since we are dealing with neutralization reactions. Some images below will help clarify things about neutralization reactions if you are lost:
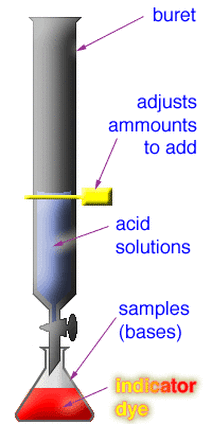
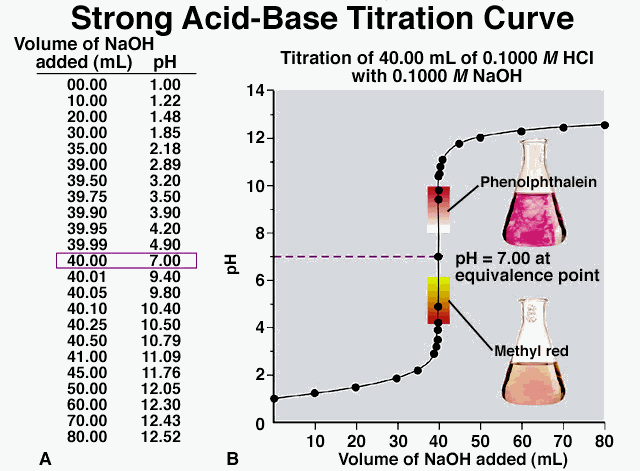
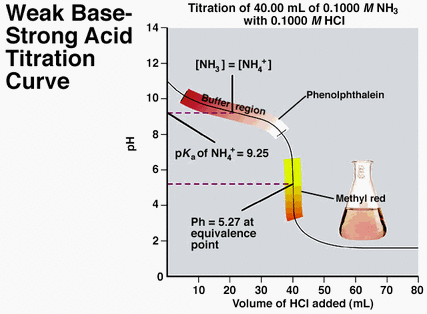
Another type of neutralization reaction is a titration. A titration is a method used in order to find out the amount of constituent in a solution by measuring the volume of a known concentration of reagent required to complete a reaction with it. This is typically done by using an indicator, which you can learn more about here. You may have performed a titration before for another type of reaction in school for a lab, and acid and base reactions work the same way! Typically for acid and base reactions, using a titration will help you determine how much acid or base is needed to neutralize the other, and also the concentration and molarity of the solution, which can be found by using the equation Molarity 1 * Volume 1 is equal to Molarity 2 * Volume 2 (M1V1 = M2V2). Typically, you will know the molarity and volume of one of the solutions (1 M for the acid and the average amount of volume it took to neutralize the base), and the volume of the other (should be how much was originally in the flask) so you can just set it up as an algebraic equation and solve for the unknown, which is the molarity. The unit for molarity is M.
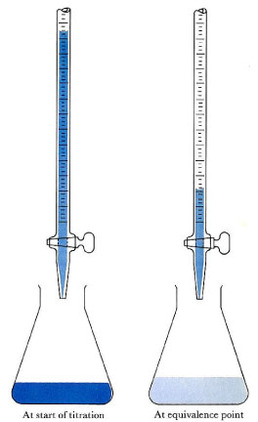
For a lab, you would first do a rough titration to see how much acid is needed to neutralize the base with phenolphthalein indicator added to it, which would be done by letting liquid out of the burette until the solution turns clear. This solution would be in a flask, which you should also weigh beforehand. You would then continue doing a similar thing for a few trials, but instead let the liquid out from the burette much slower, and then record your data, of course. In addition, you would heat the flask with the acid and base neutralized in it until salt is produced and record the final weight of the flask with the salt in it and subtract that initial weight from this to determine the weight of the salt. Below are pictures showing what a titration is, some acid-base titration curves, the materials used for the lab, along with a brief demonstration video: