Conjugate Acid & Bases
Conjugate acids and bases are a pair of substances that are a product of another acid and base being combined together.
Let's take a look at the sample reaction below:
HX (aq) + H2O (l) --> H3O (aq) + X (aq)
In the reaction above, HX is an acid and H2O is a base. X represents any element that is bound with hydrogen to form an acid and can be reacted with water. H3O is the conjugate acid of H2O and X is the conjugate base of HX. The reaction going forward is the reaction of an acid and a base. The reaction going backward is also the the reaction of an acid and a base, except in this case they must be named conjugates.
To summarize that for you:
A conjugate acid is something that is produced when a base accepts a hydrogen ion.
In the example above, H2O, which is a base, accepts a hydrogen ion from HX, which is an acid, and becomes H3O (a conjugate acid)
A conjugate base is something that is produced when an acid donates a hydrogen ion.
In the example above, HX, which is an acid, donates a hydrogen ion and becomes X (a conjugate base)
Bronsted-Lowry interactions involve conjugate acid-base pairs.
Let's take a look at the sample reaction below:
HX (aq) + H2O (l) --> H3O (aq) + X (aq)
In the reaction above, HX is an acid and H2O is a base. X represents any element that is bound with hydrogen to form an acid and can be reacted with water. H3O is the conjugate acid of H2O and X is the conjugate base of HX. The reaction going forward is the reaction of an acid and a base. The reaction going backward is also the the reaction of an acid and a base, except in this case they must be named conjugates.
To summarize that for you:
A conjugate acid is something that is produced when a base accepts a hydrogen ion.
In the example above, H2O, which is a base, accepts a hydrogen ion from HX, which is an acid, and becomes H3O (a conjugate acid)
A conjugate base is something that is produced when an acid donates a hydrogen ion.
In the example above, HX, which is an acid, donates a hydrogen ion and becomes X (a conjugate base)
Bronsted-Lowry interactions involve conjugate acid-base pairs.
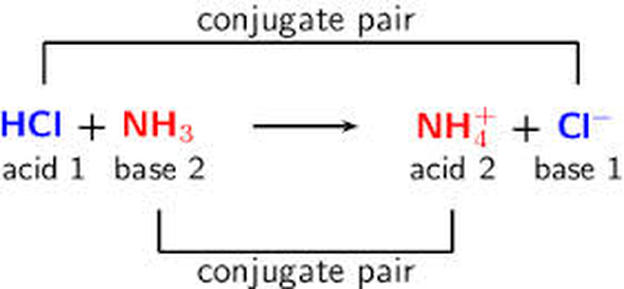
Another example of a reaction with conjugate acids and bases is shown below with lines matching the pairs for clarification: