Buffers & Indicators
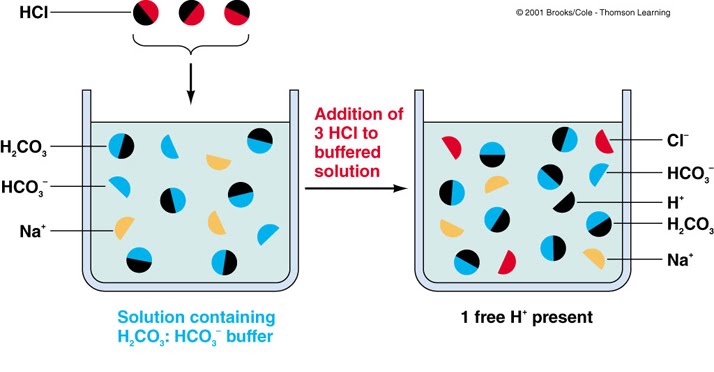
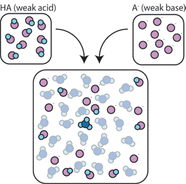
Buffers are a type of solution that resist a change in pH when an acid or base is added to it. Buffer solutions usually contain a weak acid or base combined with its conjugate acid or base. As a result, they can also be referred to as solutions that are simply responsible for combining an acid and a base. In addition, they are able to neutralize fairly small amounts of added acid and base, which results in it being able to maintain a stable pH in a solution. Buffers are especially important for reactions or processes that need a specific and stable pH. They have a working pH range and capacity that can dictate how much acid and base can be neutralized before the pH changes, and the specific amount by which it will change. Below are some examples dictating what happens in a solution with the presence of a buffer:
Buffers can also be found in many of the things that we use in everyday life. Below are some examples and how they work:
- The blood in our bodies has a constant pH of 7.4 thanks to buffers in our bodies
- Buffers are also used in our bodies in the hemoglobin complex, which helps the body utilize oxygen better
- The making of many alcoholic beverages requires buffers (related to fermentation)
- Buffers are used in the production of shampoos (examples include citric acid and sodium hydroxide)
- Buffers are used in baby powder, which helps inhibit the growth of bacteria by maintaining a pH of 6.
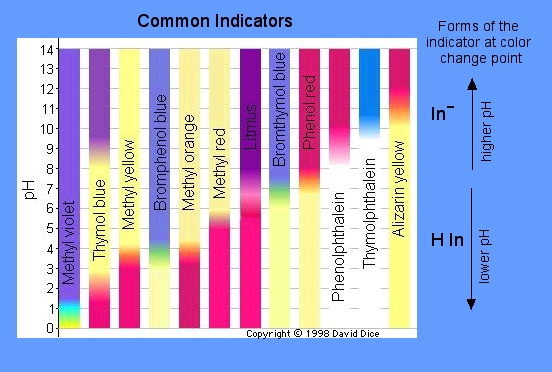
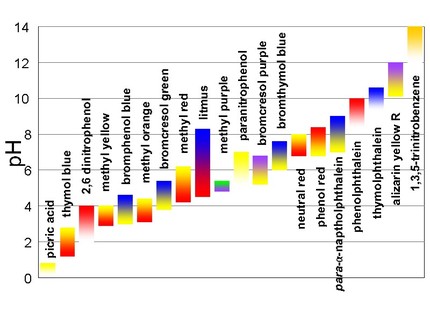
An indicator is a compound that has the ability to change color at a specific pH value or in the presence of a particular substance. Indicators can be used to monitor the acidity or alkalinity of a solution and the progress of a reaction. The indicator itself is typically a weak acid or base. An indicator does not change color from pure acid to pure base/alkaline at a specific hydrogen ion concentration, but instead changes over a range of hydrogen ion concentrations. This specific range is called the color change interval, and is expressed as a pH range. There are many different types of indicators. Below are two charts displaying some indicators and what colors they change at a specific pH:
Below are some common indicators and their specifics along with some pictures showing the color changes for different pH's:
- Universal Indicator/pH Paper - will turn a color that corresponds to the same ranges of the pH scale, meaning you will immediately know the pH from using this indicator (smooth color change); consists of water, sodium hydroxide, methyl red, etc. Also comes in drops.
- Litmus Paper - tests whether something is an acid or a base (also referred to as the litmus test). Wet litmus paper is also used to test water-soluble gases, as the gas will dissolve in water and what color the solution turns will tell you the pH. If the pH is below 4.5, the litmus paper will turn red (it's an acid). If the pH is above 8.3, the litmus paper will turn blue (it's a base).
- Phenolphthalein - a chemical compound with the formula C20H14O4 that is commonly used in titrations. It will turn colorless in acidic solutions and pink in basic solutions. Sometimes if the concentration of the indicator is really strong, it will appear to be purple.
- Methyl Orange - frequently used in titrations as it has a very clear and distinct color change. It will typically change color when it's at the pH of a mid-strength acid, so it's usually used for titrations of acids. Does not have a range of color changes like universal indicator.
- Thymol Blue - an indicator made up of a brownish-green and reddish-brown crystalline powder. It is insoluble in water, but soluble in alcohol and dilute alkali solutions. Is red with a pH below 1.2, yellow with a pH between 2.8 and 9.6, then blue above 9.6